酿酒酵母非发酵代谢调控
2007-06-17 14:48:04 来源:网络数据库 评论:0 点击:
and Schu¨ ller 1993),CAT8 (Hedges et al. 1995)], transcription of these genes remained unaffected in a mig1 null mutant (Mercado and Gancedo 1992; Scho¨ ler and Schu¨ ller 1994; Klein et al. 1999). Thus, redundant mechanisms of genetic control presumably acting at the level of gene-specific transcription factors do exist and allow full glucose repression of gluconeogenic genes, even in the absence of Mig1 (and the related Mig2 repressor, see below in this section). It should be emphasized that Mig1-binding sites can also function as activating elements in the absence of Mig1 (Bu and Schmidt 1998; Wu and Trumbly 1998). However, the corresponding activator is currently unknown. An activating role of Mig2 (see below in this section) and the zinc-finger proteins Msn2 and Msn4 involved in stress response can be ruled out (Wu and Trumbly 1998). Mutations cat4 (Schu¨ ller and Entian 1991) and ssn1(Vallier and Carlson 1994) were identified as partial suppressors of snf1 mutations and later turned out as allelic to mig1. To execute gene repression in the presence of glucose, Mig1 mediates binding to specific target promoters and simultaneously must recruit the general corepressor complex Cyc8-Tup1 (Treitel and Carlson 1995; see below in this section). A small repression effector domain at the C-terminus of Mig1 containing hydrophobic amino acids is required for interaction with Cyc8 (O¨ stling et al. 1996, 1998). Since a mig1 mutation is partially epistatic to snf1, one major function of Snf1 may be the deactivation of the Mig1 repressor in the absence of glucose (Treitel and Carlson 1995). At least in vitro, Snf1 is indeed able to phosphorylate Mig1 at four sites (serines 222, 278, 311, 381), three of which exactly match the consensus recognition motif (O¨ stling and Ronne 1998; Treitel et al. 1998; Smith et al. 1999). The importance of phosphorylation at a fifth site (serine 108; O¨ stling and Ronne 1998) is unknown. hosphorylation of Mig1 is essential to overcome its repressing function. This conclusion follows from the characterization of a mutant allele which encodes a Mig1 variant with the four serines changed to alanine. The resulting protein turned out as an efficient repressor, even in the absence of glucose (DeVit and Johnston1999). One important mechanism to deactivate Mig1 is its phosphorylation-dependent nuclear export (shown in Fig. 4). Addition or removal of glucose can alter the intracellular localization of Mig1 within minutes (DeVit et al. 1997). Central amino acids 217–400 of Mig1, containing the Snf1 phosphorylation sites, can be identified as the transport domain, with a regulated nuclear export sequence and a nuclear localization sequence. The nuclear exportin Msn5 interacts with the phosphorylated transport domain and mediates the nuclear exclusion of Mig1 (DeVit and Johnston 1999). This finding explains why MSN5 was originally isolated as a gene dosage-dependent suppressor of snf1 mutations: An increased level of Msn5 allows export of the Mig1 repressor, even in the absence of phosphorylation, and thereby alleviates the loss of Snf1 kinase. Msn5 is not an exportin specific for Mig1, since the phosphorylated form of transcriptional activator Pho4 also interacts with Msn5 (Kaffman et al. 1998). Moreover, Msn5 may also function as an importin (karyopherin) of a different set of proteins (Yoshida and Blobel 2001). Surprisingly, glucose repression of GAL1 was not altered in a msn5 null mutant (DeVit and Johnston 1999). Consequently, nuclear export of the repressor is not the sole mechanism leading to deactivation of Mig1. It can be concluded that phosphorylation by Snf1 may also affect a different pathway of repression, possibly interaction of Mig1 with Cyc8.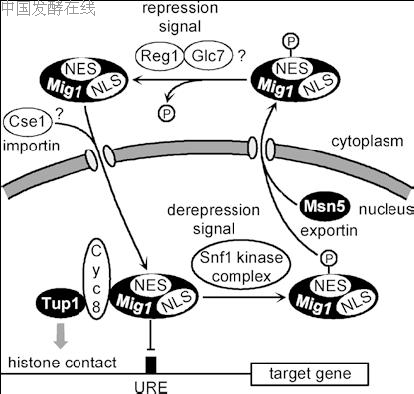
Fig. 4 Regulated nuclear import and export of the Mig1 repressor. Snf1-dependent phosphorylation of Mig1 not only triggers nuclear export but also deactivates Mig1 within the nucleus. Hypothetical interactions are indicated by a question mark. Cse1 has been suggested as the nuclear importin for Mig1 (according to DeVit and Johnston 1999). NES Nuclear export sequence, NLS nuclear localization sequence, URE upstream regulatory element
Partially redundant to Mig1 is the related zinc finger protein Mig2, which also binds to the control region of SUC2 and possibly other genes (Lutfiyya and Johnston 1996; Lutfiyya et al. 1998). However, Mig2 responds differently to regulatory signals, since no deactivation occurs by Snf1 and no regulated nuclear export can be shown.
The pleiotropic repressor Cyc8 (Ssn6) not only affects glucose-repressible genes but is also required for the negative regulation of various pathways, such as mating functions (Keleher et al. 1992), DNA damage-inducible genes (Zhou and Elledge 1992), oxygen control (Balasubramanian et al. 1993) and osmotic stress response (Marquez et al. 1998). Originally, cyc8 mutations were isolated by selection for increa
相关热词搜索:Gene regulation Transcriptional acti
上一篇:毕赤酵母表达系统
下一篇:微生物发酵处理对豆粕抗营养因子的影响
评论排行
- ·中国发酵企业数据库(4)
- ·(4)
- ·CoQ10高产菌株选育的研究进展(2)
- ·抗生素发酵工艺所用冷却塔的性能分析及处理(1)
- ·微生物菌种选育技术.rar(1)
- ·发酵生产染菌及其防治(1)
- ·赤藓糖醇发酵工艺研究(1)
- ·重组AiiA 蛋白可溶性表达及发酵条件优化(1)
- ·生物反应器设计软件_发酵罐绿色版(1)
- ·酵母粉、酵母浸粉的区别(1)
- ·雷帕霉素研究进展(1)
- ·透明质酸用途和行业概况(1)
- ·黄酒制作工艺(1)
- ·水解(酸化)工艺与厌氧发酵的区别(1)
- ·糖蜜酒精废液处理过程中产生的微生物蛋...(1)
- ·紫杉醇高产菌发酵产物的分离、纯化和鉴定(1)