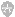Although sugars are clearly the preferred carbon sources of the yeast Saccharomyces cerevisiae, nonfermentable substrates such as ethanol, glycerol, lactate, acetate or oleate can also be used for the generation
of energy
Fig. 7 Combination of cis-acting elements upstream of a typical b-oxidation gene. It is unknown whether Snf1 directly affects Adr1 and Oaf1+Pip2. ORE Oleate response element, URS1 upstream repression site.
Conclusions: mechanisms of signal generation in carbon source control.
In S. cerevisiae, several mechanisms contribute to the signaling of an altered supply of carbon source. Glucose sensing by plasma membrane proteins Snf3 and Rgt2 is finally transmitted to the transcription factor Rgt1, which regulates various members of the HXT hexose transporter gene family (reviewed by O¨ zcan and Johnston 1999). Glucose sensing is also achieved by the membrane receptor Gpr1, which is a member of the GPCR family (G-protein coupled receptor) and affects a different signaling cascade (reviewed by Versele et al. 2001). Upon the switch from nonfermentative conditions to glycolysis, Gpr1 and its cognate G-protein Gpa2 mediate the stimulation of adenylate cyclase (Kraakman et al. 1999), leading to a transient increase in cAMP (Beullens et al. 1988) and subsequently to the activation of cAMP-dependent PKA. High PKA activity represses transcription of genes driven by CSRE motifs (Zaragoza and Gancedo 2001) and, thus, inhibits the utilization of nonfermentable carbon sources. In addition to glucose sensing by proteins of the plasma membrane, the cytoplasmic concentration of glucose may also act as a signal trigger (Teusink et al. 1998). Using continuous cultures with varying dilution rates and glucose concentrations, it could be demonstrated that glucose regulation of SUC2 transcription correlates with the extracellular or intracellular glucose concentration, but not with the metabolic glucose flux (Meijer et al. 1998). However, the mechanism of intracellular glucose sensing and its relation to the onset of glucose repression is unknown. A signal cascade which finally affects the intracellular localization and/or the phosphorylation state of the Snf1 protein kinase complex has not yet been defined. Under repressing conditions, hexokinase PII (and presumably even the differently regulated isoenzyme PI; Rose et al. 1991), together with the Reg1/Glc7 phosphatase complex, may deactivate Snf1.
The regulatory importance of Snf1 for almost all pathways using alternative carbon substrates creates the problem of triggering the correct signal which is required in a certain situation. Specificity may be guaranteed by the existence of secondary signals (possibly inducing metabolites or transport of a substrate) which could act either upstream or downstream of Snf1. Although activation of Snf1 correlates with a high AMP/ATP ratio (Wilson et al. 1996), additional mechanisms leading to derepression must exist. The functional versatility of Snf1 in various regulatory pathways clearly argues against a single (and simple) trigger, such as energy charge. Indeed, by characterizing chimeric Snf1 variants containing sequences from yeast and the mammalian AMP-activated kinase, evidence was obtained for an alternative, non-AMP-dependent, pathway responsible for activation of the protein kinase (Daniel and Carling 2002). Importantly, glucose is certainly not the sole regulator of carbon source utilization. Structural genes of gluconeogenesis driven by CSRE motifs are similarly repressed by glucose, maltose and galactose (Scho¨ ler and Schu¨ ller 1993). Recent findings suggest a two-step model for transcriptional derepression of gluconeogenic genes (Vincent et al. 2001b). While cytoplasmic phosphorylation of Snf1 may be sufficient for the activation of SUC, MAL and GAL genes, subsequent nuclear import of the kinase complex mediated by Gal83 is required for CSRE-dependent gene expression. It has been suggested that glucose-6-phosphate as a metabolic intermediate common to the catabolism of glucose and galactose could function as a signaling molecule, affecting Gal83 and its intracellular localization.
Acknowledgements Work in the author’s laboratory was supported by the Deutsche Forschungsgemeinschaft. I thank G. Schittek and S. Schade for excellent support with the design of figures.
Reference (略)