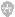1000X IPTG
0.5M IPTG 1.19g IPTG
up to 10 ml wih sterile Q
filter and store at -20°C
Buffer A
50 mM Tris 7.9 25 ml 1M Tris pH 8.5*
50 mM dextrose 4.5 g dextrose
1 mM EDTA 1 ml 0.5M EDTA
up to 500 ml with Q
*Check pH and bring to 7.9 as the dextrose will reduce the pH.
Store at room temperature. For some steps add Lysozyme to a
final concentration of 4 mg/ml.
Buffer B
10 mM Tris 7.9 5 ml Tris pH 7.9
50 mM KCl 1.86 g KCl
1 mM EDTA 1 ml 0.5M EDTA
0.5% Tween 20 5 ml 50% Tween 20
0.5% NP-40 5 ml 50% NP-40
up to 500 ml with Q
Store at room temperature and add PMSF to a final concentration of 1 mM just prior to use.
Buffer C
50 mM Tris 8.0 50 ml 1M Tris pH 8.0
50 mM KCl 3.72 g KCl
1 mM EDTA 2 ml 500 mM EDTA pH 8.0
50% glycerol 500 ml glycerol
0.5% Tween 20 5 ml 50% Tween 20
0.5% NP-40 5 ml 50% NP-40
1 mM DTT 2 ml 0.5M DTT
1 mM PMSF 10 ml 100 mM PMSF
up to 1 liter with Q
Buffer D
50 mM HEPES 7.9 50 ml 1M HEPES pH 7.9
50 mM KCl 3.72 g KCl
5% glycerol 100 ml 50% glycerol
1 mM EDTA 2 ml 0.5M EDTA 8.0
0.5% Tween 20 10 ml 50% Tween 20
0.5% NP-40 10 ml 50% NP-40
1 mM DTT 2 ml 0.5M DTT
1 mM PMSF 10 ml 100 mM PMSF
up to 1 liter with Q
Procedure
• Streak out the E. coli strain carrying the Taq polymerase gene on an LB amp plate and innoculate a 5 ml overnight culture with a single colony.
• Innoculate a 1 liter culture of LB-amp with the 5 ml of overnight culture and grow to an A600 of 0.2. Add IPTG to a final concentration of 5 mM and culture overnight.
• Spin down the culture and resuspend the pellet in 200 ml of Buffer A (ice cold).
• Spin down the E. coli as before and resuspend in 50 ml Buffer A containing 4 mg/ml lysozyme. Incubate at room temperature for 15 minutes.
• Add 50 ml Buffer B and incubate at 75°C for 1 hour.
• Chill on ice and spin in the GSA rotor at 8,000 rpm for 15 minutes.
• Transfer the supernatant to a beaker on ice. Measure the volume and add pulverized ammonium sulfate slowly to a final concentration of of 0.164 g/ml (30% saturation). Continue to stir for 30 minutes.
• Spin down the precipitate by spinning in the SA-600 rotor at 13,000 rpm for 30 minutes at 4°C.
• Transfer the supernatant to a clean beaker on ice, measure the volume and add ammonium sulfate to a final concentration of 0.181 g/ml (60% saturation). Stir as before for 30 minutes on ice.
• Spin down the precipitate in the SA-600 rotor at 13,000 rpm for 30 minutes at 4°C and resuspend in 10 ml Buffer D.
• Equilibrate a DEAE-sephacel column (1.5 cm diameter, 5 diameter height, 9 ml bed volume) in Buffer D.
• Load protein and wash with 3-5 column volumes of buffer D.
• Elute in 20 ml buffer D containing 0.5M KCl and collect the first six 3 ml fractions.
• Dialyze fractions 2,3,4 against 1 liter of Buffer C containing 50% glycerol for 4-8 hours and repeat. This should be done on ice in the cold room.
• Freeze in small aliquotes and store at -80°C.