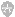Description
For protein concentration, gas pressure is applied directly to ultrafiltration cell. Solutes above the membrane"s molecular weight (MW) cut-off are retained in cell, while water and solutes below the cut-off pass into the filtrate and out of cell.| 8050 | 8400 | |
| Cell capacity (ml) Stirred minimum volume¹ (ml) Membrane diameter (mm) Effective membrane area (cm ²) Hold-up volume² (ml) |
50 2.5 43 13.4 0.5 |
400 10.0 76 41.8 1.5 |
| Maximum operating pressure: 75 psi (5.3 kg/cm²) Relief valve setting: 90 psi (6.3 kg/cm²) Maximum diafiltration operating pressure: 55 psi (3.9 kg/cm²) | ||
| ¹ Minimum working volume. ² Non-recoverable volume (below membrane surface). | ||
Membranes
- YM10 Ø43 mm (for 8050), Amicon #13622, 10 pack: $108
YM10 Ø76 mm (for 8400), Amicon #13642, 10 pack: $139 - Prewash: float membrane with glossy side down in beaker with distilled water for at least 1 hour changing water 3 times. All membranes are pretreated with glycerine to prevent drying, YM membranes are treated with sodium azide as a preservative.
- Cleaning: rinse with 0.1M NaOH, followed by thorough flushing with distilled water. For strongly absorbed protein, soak in 0.1% protease solution and rinse thoroughly.
- Storage: for reuse, store membranes in 10% ethanol water solution at 4°C.
Assembly
- Place membrane in holder, shiny side up; then place O-ring on top of membrane. Gently push O-ring down so that it contacts and seats membrane evenly in bottom of holder. Handle membrane by its edges to avoid scratching or contaminating surface.
- Fit membrane holder into cell body, aligning tabs on sides of holder with slots in base of cell body.
- Invert cell body and membrane holder; screw base firmly into bottom of cell body. A definite "stop" will be felt when base and body are fully engaged. Top of membrane holder will be flush with bottom of slots in cell body.
- Push filtrate exit tubing onto exit spout of membrane support.
- Place stirrer assembly into cell body. When properly installed, arms of stirrer assembly will rest on small ridge inside top of cell body.
- Introduce sample into cell.
- With a twisting motion, push cap down onto cell body, orienting gas inlet port on cap opposite filtrate exit port on holder. If cap assembly does not slide easily, lubricate O-ring lightly with petroleum jelly. Do not allow petroleum jelly to contact membrane!
- Set pressure-relief valve to horizontal (open) position.
- Slide cell into retaining stand, fitting ring on cell base into hole in stand. Flattened edges on bottom flange of cap ensures that cell is inserted properly and prevents rotation of cell once inside stand.
- Turn pressure-relief valve to vertical (closed) position.
- Attach gas pressure line.
Operation
- Place cell on magnetic stirring table.
- Connect inlet line to regulated gas pressure source (Nitrogen gas is recommended for pressurizing cell! Use of compressed air can cause large pH shifts due to dissolution of carbon dioxide. With sensitive solutions, oxidation can also occur, leading to other potential problems.)
- Hold cell steady on the stirring table and pressurize according to instructions in membrane package. Generally 55 psi (3.7 kg/cm2) is optimal, maximal 70 psi (4.7 kg/cm2) nitrogen gas pressure. Cap assembly moves upward, forming a secure lock with retaining stand once system is pressurized.
- Turn on stirring table and adjust stirring rate until the vortex created is approximately one-third the depth of the liquid volume.
- Monitor concentration. Do not allow retentate to run through.
- Collect permeate.
- Highly viscous solutions filter slowly, as do solutions containing particulate matter, such as colloids. Where the viscous agent (sucrose, glycerin, etc.) is to be removed, flow can often be increased by predilution.
- Prefilter or centrifuge any solution containing particulate matter, such as cell debris or precipitates.
- To maximize recovery of retained substances, continue stirring for a few minutes after depressurization. This will resuspend the polarized layer at the membrane surface.
- When finished, turn off nitrogen pressure source and stirring table.
- Vent pressure inside cell by slowly turning pressure-relief knob to horizontal position. Push cap down, then slide cell out from retaining stand. Note: Overly rapid depressurization can cause the membrane to buckle up and rupture.
- Using a twisting motion, remove cell cap and the magnetic stirrer assembly. Always remove the cell top with the pressure-relief valve set to the horizontal (open) position. Removal in vertical (closed) position can create a partial vacuum which can rupture the membrane! Pour out solution.
- Disassemble cell, then wash all components with a mild detergent/water solution, then rinse thoroughly. Caustic cleaning solutions may damage the anodized aluminum retaining stand. Leave ultrafiltration cell disassembled whenever it is unlikely to be used for several weeks.
Limits
- max. cell operating pressure: 75 psi (5.3 kg/cm2)
- max. membrane operating pressure: 70 psi (4.7 kg/cm2)
- pressure-relief valve preset: 90 psi (6.3 kg/cm2)
- Although brief exposure to higher temperatures is possible, do not operate cell continuously above 85°C (185°F).
- pH 2 to 10
- Do not use the following chemicals: Ketones (including acetone), Aromatic hydrocarbons (including toluene), Cellosolvers, Halogenated hydrocarbons, DMF, Aliphatic esters, DMSO, Polar Aromatics. Note:The spring in the pressure-relief valve is not compatible with 0.1N NaOH.
Sterilization
Amicon stirred ultrafiltration cells can be autoclaved at 121°C (250°F) for 30 minutes. They are compatible with standard sterilizing gas mixtures, 70% ethanol, isopropanol or 5% formalin. CAUTION: Tighten the base partially before autoclaving.Maintenance
- Replace O-rings at the first sign of damage or wear.
- If installation or removal of cap assembly becomes difficult, lubricate cap O-ring with a small amount of petroleum jelly. Do not allow petroleum jelly to come in contact with membrane!
- Check stirrer periodically for rough edges that could possibly damage the membrane.
- Replace transparent body immediately should it become cracked or crazed.
Troubleshooting
Little or no filtrate obtained:- Rotate pressure-relief valve to check for pressure. If not pressurized, check nitrogen source and regulator.
- Make sure glossy side of disc membrane faces up.
- If sample solution is highly viscous due to microsolutes, either dilute or diafilter to increase flow rate.
- Check membrane holder and filtrate port for blockage.
- Check membrane for lesions, scratches, or roughness.
- Make sure correct membrane type is being used.
- Check stirrer assembly to ensure that stirring bar is not contacting the membrane surface.
- Be sure lower O-ring rests entirely on the membrane peripheral surface.
- Check O-rings for nicks and cuts.
- Make sure membrane support is seated properly, and that the base is screwed in firmly.
- Make sure O-rings are not squeezed out of slots.
- Check fittings on gas inlet tubing for correct order and position.